PICTURES COMING SOON
Gallium: a weird liquid metal in your hand! It’s as shiny and mobile as mercury but so safe… unless you’re a Coke can. It’s a liquid wire to carry electricity. It acts like ice on freezing. With a simple AAA cell you can make it take extremely different shapes. It’s easy and inexpensive to buy, for much fun.
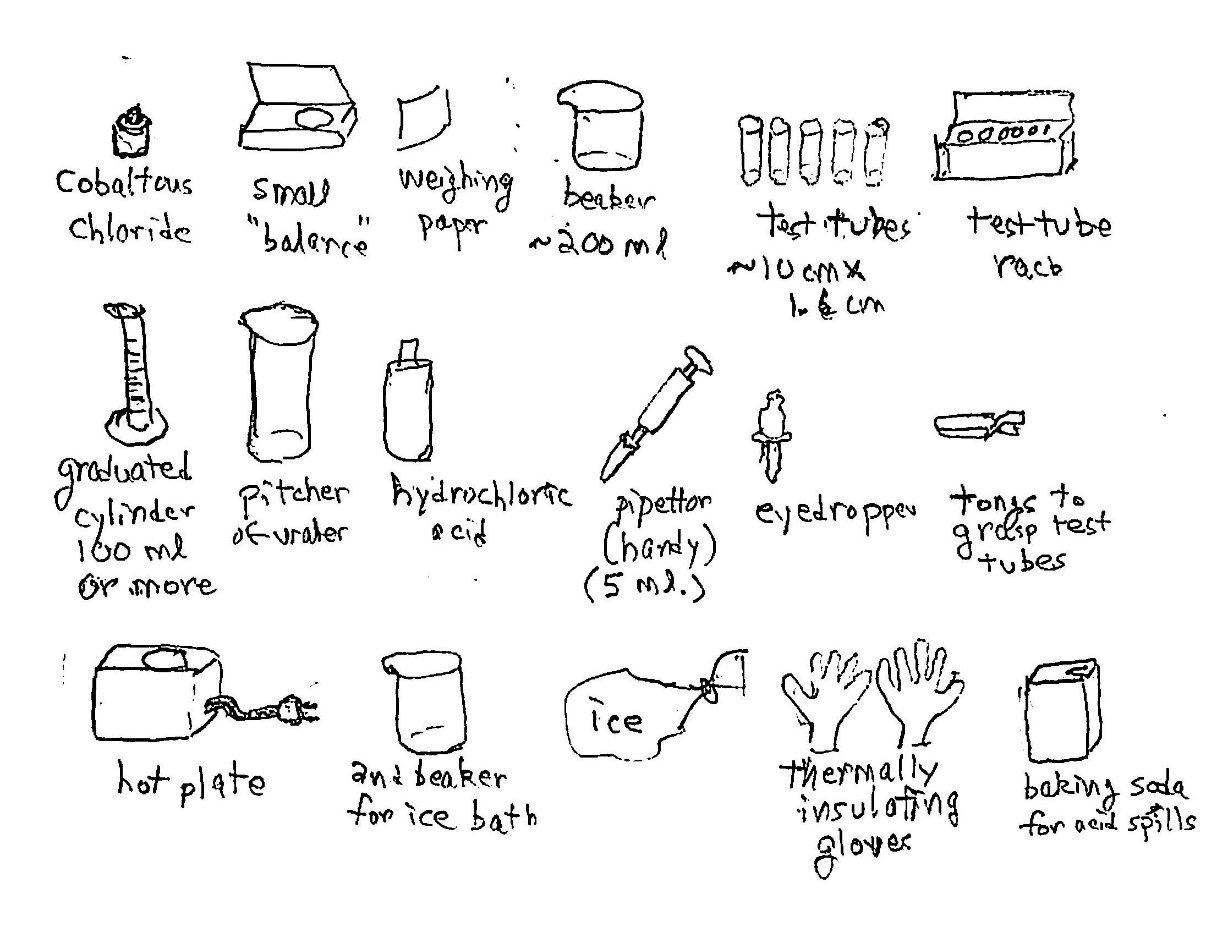
Equipment and supplies: For many parts of this demo It depends on how many of the 6 parts of the whole demo you wish to do. Foremost, you need gallium ($14 or more; see below). Get some simple containers, cups or chemical beakers, to hold warm or cold water. Get a piece of glass (careful of the edges) or a glass bottle for demo 2. For demo 3 get an empty aluminum can and a piece of sandpaper. For demo 4 get a small, clear, rigid tube – glass or plastic. For demo 5 make a small trough, even in modeling clay, and get the use of an electronic multimeter ($10 or more) to measure electrical resistance. For demo 6 get a beaker, some sodium hydroxide ($10, or less if you pick the lye crystals out of some Drano carefully, not with bare hands), some wire, a piece of aluminum (maybe even a bit of aluminum foil), and a AAA cell.
Many of us, chemists and members of the general public, remember mercury, the liquid metal. You could pour it out, even onto your hand, as a beautiful, silvery, shimmering liquid. While you can still do that, people will look askance at you for the potential health hazard. There is such a hazard but almost solely if you let certain bacteria at it in oxygen-free environments to make astoundingly toxic methyl mercury or dimethyl mercury. So, avoid the problem and get some gallium. I bought some several years ago along with also fascinating bismuth metal, for a total of $55. You can get it from Luciteria.com, where you can get almost any chemical element! You can get 20 grams for about $14.
Demo 1: Melt it, even in your hand: The gallium will sit in the container as a silvery solid like any nice metal. However, warm it up a little and it melts. If you’re patient and your hands are not cold, it will melt in your hand. Its melting point is 30°C, which is 86°F. If you’re less patient, warm up some warm and insert the container.
Demo 2: The shimmer and the streak: Carefully pour some out onto your hand. No worries. It’s essentially totally nontoxic. Roll it around and let it slosh around. Try not to spill any; it will leave a gray streak on lots of surfaces, even on your hand. The streak is readily washed off with a little soap and water. You can paint on glass with gallium, with a little practice. Get some gallium on your finger and rub it onto a clean glass surface. I did this on old wine bottles. Only a little suffices to create a fine, silvery mirror.
PICTURE(S)
Demo 3: Eating a coke can! Find an empty coke can or other aluminum can. Empty, I say, for reasons that become clear. Have your gallium liquid. Take a bit of sandpaper and scrape an area on the lid of the can. Deposit some liquid gallium on the area. Come back in an hour or two. The gallium will look odd and the can, more so. You can now push your finger easily through the lid! Gallium atoms have diffused into the aluminum metal, moving on their own just as a drop of food coloring diffuses slowly into still water. Gallium is a chemical analog of aluminum – it shares many of the same physical and chemical properties, but its crystal structure as a solid is spaced differently from that of aluminum. The presence of gallium pushes the crystals of aluminum apart, massively weakening the lid (yes, crystals, as are all solid metals in many small places). You’ll also notice that the decay of the aluminum lid extends well beyond the place where you applied the gallium; the diffusion kept going.
PICTURE
Demo 4: It’s slow to freeze and it does an odd thing: Gallium is one of the very few liquids that expands on freezing, just as water ice does. As also with ice, you can demonstrate this by putting a little bit of it as a liquid, a few grams, into a tube and letting it cool, say, in a bath of cool water. If you have a thermometer you can try baths of different temperatures. You’ll find that it takes a bath a number of degrees below the nominal melting point of 30°C for gallium to freeze. It will “supercool” and then suddenly start turning solid when you’ve pushed supercooling too far. The same supercooling phenomenon occurs in water. Some clouds have water droplets cooled far below 0°C. An airplane flying through such a cloud can collect these droplets that instantly become ice, and that’s usually a growing problem! Also, you can mark the tube at the height of the liquid and then see that it’s higher when the solid forms. The expansion of the liquid on freezing can create great force, as is readily shown by freezing water inside a metal container filled to the top (a soft drink so frozen has additional things going on, though it also explodes).
Demo 5: The liquid is a fine electrical conductor: Most metals conduct electricity much more poorly as liquids than as solids. The disorder of the locations of atoms in the liquid phase causes the electrons moving through it to scatter. They lose momentum and it takes more force, more voltage, to move a current. To see this, solidify some gallium in a “boat” shape – that is, some little trough with closed ends. Take a simple electronic multimeter that you’ve set to the setting of resistance (ohms, Ω). Touch the two probe tips to the two ends of the solid gallium and record the resistance in ohms. Now, melt the gallium in the trough by any handy means, even a hair dryer on low heat held at a distance so as not to splatter the liquid. When the gallium is liquid, repeat the measurement. It’s lower!
PICTURE
Demo 6: Gallium has a huge surface tension: When you put a drop of water on a surface that water doesn’t wet, such as clean glass or most plastics or some plant leaves, it forms into a ball, a bead. The attraction of the water molecules for themselves is far stronger than their attraction for the molecules of the surface. The best way to reach the lowest energy state is to stay as compact as possible, toward a spherical shape. That reduces the surface created. Surface tension is a measure of how much new force is needed to expose more surface. There’s a lot to explore in that concept. For now, let’s just note that gallium has seven times higher surface tension than water! Hmm. So why does it flow nicely over my hand? Well, when it’s exposed to air, gallium combines with oxygen gas in the air to make an extremely thin layer of gallium oxide, Ga2O3. Aluminum also makes an oxide layer, as you might expect from Ga and Al being in the same column of the periodic table of the chemical elements. Anyway, we can eliminate that layer.
Here we go: Get a chemical beaker. Pour in some water that is warm enough to keep the gallium liquid. Dissolve a bit of sodium hydroxide, known as lye, in the water. Do this carefully, since lye is corrosive and hazardous to human tissue (on your hand, it will first just make it slippery because it breaks down fats around your cells. Don’t leave it there. Don’t let it get to your eye! Wash it off; wash your eye with running water for several minutes if you get any lye in it. To continue, make a simple electrical circuit. Attach a wire to a piece of aluminum and suspend at least part of the aluminum in the liquid. Run another wire to the liquid gallium at the bottom of the beaker. Now, touch the two ends of the wires you have outside the liquid to the two ends of a 1.5 volt battery, such as a AAA cell. One of two things will happen. If you put the wire that runs to the gallium on the bottom of the AAA cell, you have made the gallium negative electrically. That chemically reduces the oxide coating to pure gallium. It then exhibits its maximum surface tension and really balls up. Now, switch the leads, making the gallium positive. It gets oxidized… .and it spreads out into fantastic shapes called fractals. It spreads even though the oxide coating is something of a restraint. The coating keeps getting dissolved by the lye, allowing the metal to spread!
Picture
End notes – some history: Where did the name gallium come from? The existence of gallium as a chemical element was predicted by the great Russian chemist Dmitri Mendeleev in 1867. He had data from the world chemistry community (hard to get in pre-Internet days!) about 48 chemical elements, including the relative mass of the atoms in each element. He put them in a 2-by-2 chart according to repeating properties. There was a gap below aluminum, so he boldly predicted there was a metallic element acting a lot like aluminum chemically with an atomic mass of about 68 with a density of 5.9 grams per cubic centimeter. In 1875 the fine French chemist Paul-Émile Lecoq de Boisbaudran used the relatively new method of spectroscopy to determine there was another, distinct chemical element in some metal samples. He purified it and there was gallium. It’s atomic mass of 69.7 was close to Mendeleev’s prediction, and so was its density of 5.91 grams per cubic centimeter. Being French, Boisbaudran honored his home country by giving the element a name derived from the Latin name of France, Gallia. Boisbaudran is also credited with discovering the elements samarium and dysprosium. His name is pronounced as bwah boh drahn, or more precisely in international phonetic symbols that I haven’t copied here. The “r” is lightly trilled and the “ah” is breathy.











 VIDEO TO COME
VIDEO TO COME