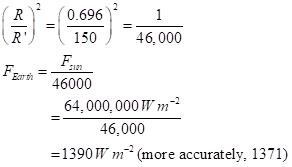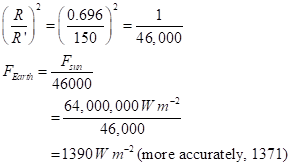MORE PICTURES TO COME
Adding up colors: We’re all used to mixing paints or crayon colors and getting darker and darker colors. Adding colors gives us a very different experience, and we only need three different colors, red, green, and blue. This happens all the time in digital displays; there are simple ways to see this in near-microscopic detail. We can also make our own color mixer, or color adder, with three LEDs.
Let’s mix colors, both adding and subtracting them
Color perceived as a mix of three primary colors: We humans mostly have three different color-sensing cells in our eyes, in our retina. Each one responds to a different range of colors (wavelengths) of light. You can think of them as responding, very broadly, to red (one type of cone), to green (another type), and to blue (the third type). We get a certain mixture of “colors” in light as it is emitted by various sources – the sun, a lamp, … – and possibly reflected or transmitted by various surfaces – a leaf, a colored windowpane, … Our brain interprets any given mix of colors of light as a particular overall color or hue. We all know the experience and we may know a lot of different hues by name. There is also value or lightness, denoting how bright overall the light us, and there is saturation, denoting how diluted the color is by mixed-in white, such as pink being a mix of pure red and some white. There are some tricky parts, explained in a number of books or websites, such as hues looking different depending on nearby hues in a scene, but let’s leave that here. In any event, we can create a color as a hue, a saturation, and a value by getting three different pure color sources – red, green, and blue – and adjusting the brightness of each. Again, there are tricky parts because sources with very narrow bands of color miss some features – it’s why some color prints can’t be made to look exactly the way they appeared to our eyes; that, too, is another story. Here, we are going to see how three different colored light sources can make a great range of colors.
Short notes: Some people lack one or more color receptors. We call them color-blind, or, more appropriately, color-confused. They see all the colors but they seem different; some of them mix up greens and reds, for example. Some people have had their corneas – front of the eye – removed surgically, and they see ultraviolet light that the rest of us don’t. Bees also see ultraviolet. The mantis shrimp has 13 different color receptors but only sees a small range of hues; a lot of the receptors register other properties of light, including its polarization (more on that in other demos or experiments).
Several methods of adding colors: Adding colors is done all the time in a digital color display, though it can be hard to see it happening, so, let’s mix them under our control, with three (or more) ways of doing this:
— Lighting up 3 light-emitting diodes (LEDs), one each of re, green, and blue. We can vary the relative brightness of each LED to create different colors. For some of you, it may seem counterintuitive that adding red, green, and blue light makes white, while mixing red, green, and blue pigments as paint or as crayon streaks makes black, or nearly so depending on the crayon and paint exact colors. We can build a color mixer from simple electronic parts and a supporting box. We’ll get to the details below.
— Watching a color monitor of a computer add up colors from its many small picture elements, or pixels. Viewed from a normal distance, the colors from individual red, green, and blue (RGB) pixels blend in our eyes to make any specific color. We’ll need a camera with a close-focusing or macro lens to be able to see the individual pixels, which have to be quite small.
— Browsing to a website such as http://www.cknuckles.com/rgbsliders.html, where we can use our mouse to set the levels of R, G, and B light on a big square in our monitor.
First method: adding the output of 3 LEDs:
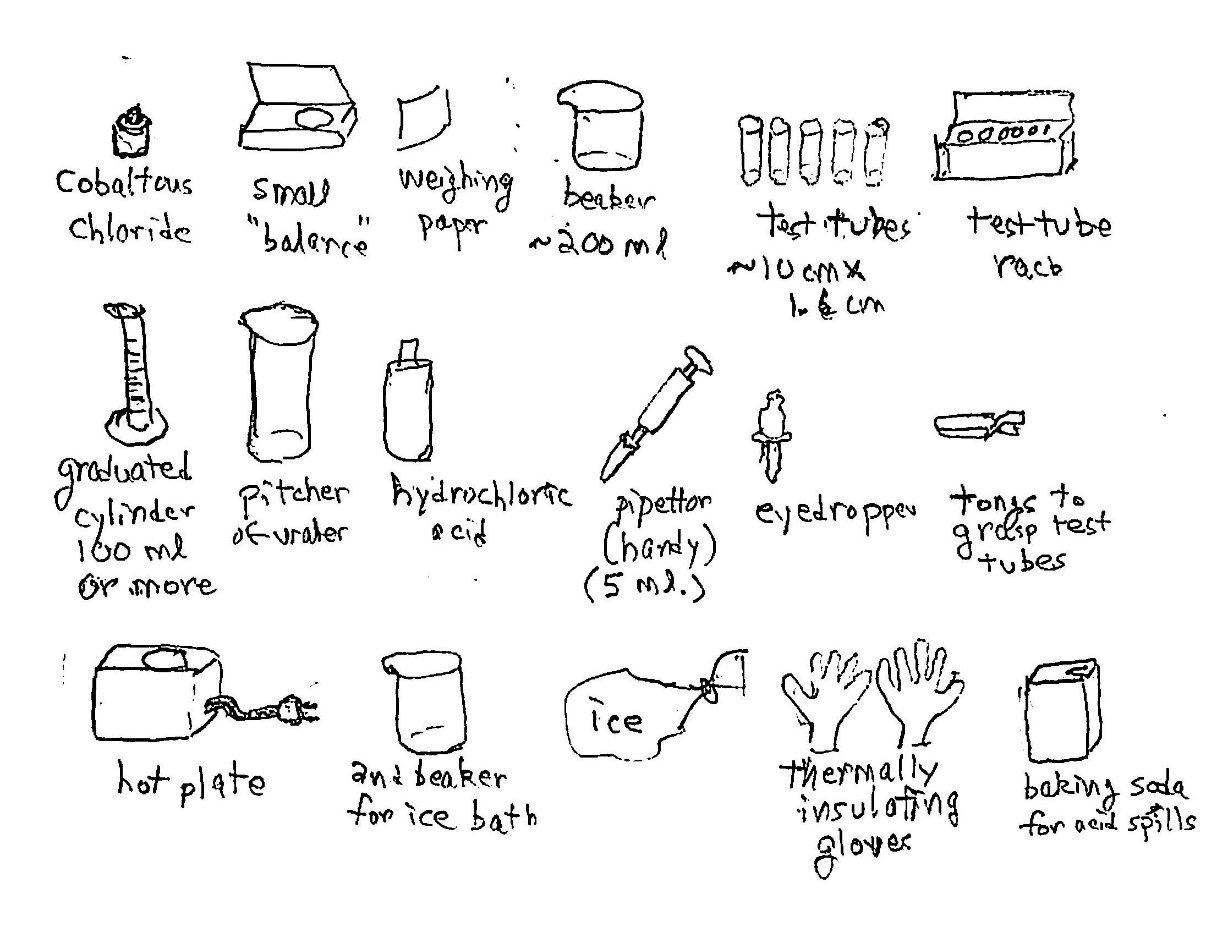
Equipment: We’ll build a small box with the LEDs, a battery for power, and controls for the level of output of each LED, with a small area where we view the colors mixing. We need:
* One each of a red, a green, and a blue LED, all of comparable light output at maximum. There are many places to buy these. For these and for all kinds of electronic parts, I recommend DigiKey.com. Get LEDs that have a peak current of 20 to 40 millamperes (mA). You’ll use the battery, below, to create currents to light them up, and electrical resistors to control just how much goes though each LED – that is, how bright each one is;
* A 9-volt battery and associated stuff: a “lead clip” that snaps onto the terminals to provide two wires you can attach to the rest of the circuit, and a handy clip to hold the battery to the box you’ll build; it simply snaps about the battery body;
* A resistor of fixed, low resistance for each LED, to limit the peak current so that you won’t burn out your LED. Suppose your LED has a peak current I of 20 mA or 0.020 amperes. The red LED has a voltage “drop” of about 2.2 volts, just to start current flowing (and it varies only a little with current). That leaves 6.8V to drop across the resistor. From Ohm’s law (which you can look up, if it’s not familiar to you), the resistor you need has the value R0 = V/I, or 6.8V/0.020A = 340 ohms (symbol Ω). It’s not critical to have that exact value, so choose common and very inexpensive 330 Ω resistor. It will have little current through it to heat it up, so you can use a low-power resistor, 1/8 watt or ¼ watt, though any power rating will work if you have a resistor handy. For the green LED, the voltage drop will be about 2.8V and for the blue about 3.3V. You can use resistors for these with slightly smaller values, but it’s not critical;
* A variable resistor or potentiometer for each LED, to vary the current. You want its highest resistance to limit the brightness to, say, 1% of maximum. So, you need a potentiometer rated at 100 times higher resistance, or about 33,000 Ω. You won’t find one for sale, so pick one that’s relatively close – say, 50k Ω (kilohms). There are several kinds of potentiometers that vary in “taper,” which is how rapidly or in what pattern the resistance varies with the turn of its dial. A convenient taper is called audio – it starts changing slowly and then more rapidly as you move the dial. The proportional increase in resistance is about the same along each fraction that you turn the dial. This will make the changes in brightness fit the sensitivity of our eyes. We notice relative changes in brightness, not really absolute changes. This will make the effects very clear;
* An on-off switch for convenience, as an alternative to pulling the lead clip off the battery each time to shut it down (and an alternative to leaving the color mixer on and running down the battery). A simple single-pole single-throw switch will work. Single-pole means that we are only going to control one wire, as the circuit diagram will show. Single-throw means that there is only one on-position and the other is an off-position. Be sure to get a toggle switch that stays in the position you want; there are also momentary-contact switches that revert the moment you stop pressing them;
* A set of “hardware” to mount all this stuff – particularly a nice clear or translucent plastic box to hold everything and a piece of thin, stiff, insulating board (“perfboard”) on which to mount the LEDs and the resistors. A full list is at the end;
* A piece of thin, diffusing plastic to “mix” the lights on a space you’ll use to observe the results;
* Some side-cutters, pliers, solder, and a soldering iron to make all the connections. Side-cutters let you cut wires and the leads of the resistors and the LEDs to handy lengths. They can also be used, with some skill you readily acquire, to strip insulation off the battery clip leads; otherwise, use a small, sharp knife with care. Needlenose pliers are handy for holding the LED leads while you solder to them. They act as “heat sinks” to much reduce the heat going into the LED and possibly damaging it. It’s handy to tie their handles together with a stiff rubber band so that the jaws stay clamped and the whole setup is stable while you solder. Learning to use a soldering iron is a handy skill. Be prepared to get a small burn or two, almost as a right of passage.
The pictures here show stages of assembly. There’s also a standard schematic of the electrical circuit. Getting familiar with conventional symbols is very useful

Using the device: Hey, play with it. Change the amounts of each LED’s light output and look where the light patches overlap. You can readily see that mixing approximately equal intensities of red and green gives us an even brighter yellow. Mixing red and blue gives us a pretty magenta, or a reddish or a bluish version of magenta. Mixing all three colors gives us an approximate white. I say “approximate” because the colors of common LEDs don’t quite fill in all the color space. In any event, this is an inexpensive device that is handy for demonstrations.
Method 2: looking at a color monitor on a computer to see the tiny colored pixels that blend in our eyes
Equipment: There are several ways to do this. I’ll present one that I found handy. Needed: a computer displaying colors in any desired pattern on its monitor ($0, if you have a computer; I assume you do, since you’re likely reading this on the screen); a digital camera such as a single-lens reflex or a newer mirrorless camera. You’ll want one that displays what it is seeing on an LCD screen on its back; a macro lens that lets the computer focus very close on a small area of the monitor. I use a Canon 60mm macro that will go to an enlargement of 1-to-1 (some macro lenses do much less, such as 1-to-5 even; you’ll want at least 1-to-2).
How to see the pixels: Pixel, is, of course, short of picture element. The computer monitor is covered with a million or so pixels. Let’s look at some in detail. Get an interesting color-varied display on the monitor. I put a hard surface such as a clipboard on my laptop computer’s keyboard so that I won’t be pressing keys and going off-scene. I set my macro lens to focus as closely as possible; that gives it 1-to-1 enlargement. I put the camera on the clipboard and click the control that shows the camera image on the camera’s back screen. Then I move it closer or farther from the screen so that the pieces of the screen, the pixels, are in focus. If you do the same you’ll see a grid of many very small and tall rectangles. Push the control on your camera that lets you magnify the image. Zow! You see all the patches of red, green, and blue that make up the monitor screen. You may want to save the image by taking the picture. You can move the camera around to various parts of the scene on the monitor that differ in color, hence differ in how much the red, green, and blue pixels light up.
PICTURES or video
How pixels work: On my laptop monitor there is just a repeating array of red, green, and blue pixels across the screen horizontally, with about 766 arrays stacked on top of each other vertically. Under commands from the graphics card, each red, green, or blue pixel is allowed to pass a fraction of the white light behind them. The balance of R, G, and B determines the color. Note that the pixels are not like LEDs; they do not actively emit light. They only passively pass part (or even all) of the white light behind them. Each pixel has its own precisely made color filter behind it… a better match to the R, G, and B of our visual cones than is the set of colors of the R, G, and B of the LEDs in the first method. The way that the pixel light transmission is controlled is shown brilliantly in a video by the Engineer Guy, XXX, as he takes apart a computer monitor. It’s well worth watching, more than once. To continue, at our normal viewing distance from the monitor the pixels’ light all blends into one part of our visual image, so that our eyes – and our brain – interpret the mix as a specific color, a hue, a saturation, and a value.
Method 3: find a website that lets us set the RGB color mix in a big patch on the computer screen
Equipment: Just your computer. Navigate to http://www.cknuckles.com/rgbsliders.html . You’ll see three “sliders” that you can drag with your mouse to set any level of R and G and B
CAROUSEL
Play with it. Of course, you don’t get to see how the individual pixels light up, but you can have a lot of fun wit the colors. You’ll see advertising claims that monitor model X can display 16 million or so colors. It can display that many different mixes of RGB colors (though maybe not fully reliably – a slight voltage change might make a 1% change in all intensities, which is about the same as smearing out 3 of the 256 different levels of each R, G, and B pixel). Human vision is not that finely attuned. We can discriminate about 20,000 colors, and, among these, maybe 200 different hues, the rest of the variation being in saturation and value. Some people say we can see 1 million colors; don’t count on it, and we don’t need it; we have very rich color vision, in any case!










 VIDEO TO COME
VIDEO TO COME