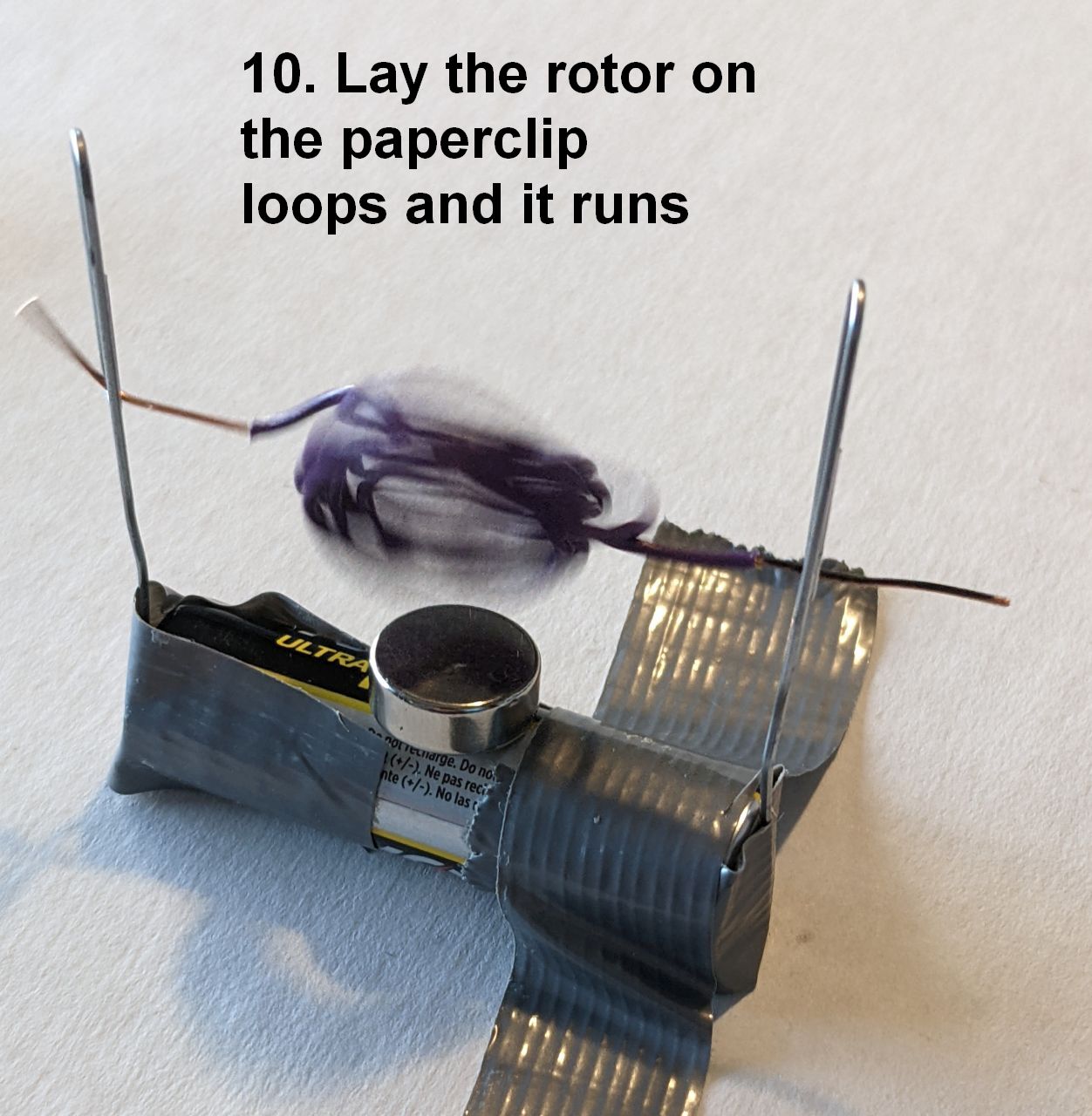
PICTURES COMING SOON
What does Isaac Newton teach us with an intriguing “toy,” Newton’s cradle. The quantity of motion, or momentum, is conserved. When steel balls collide, there are many possible patterns of which ones go and how fast. It’s addictive to try many patterns.
Newton’s cradle: momentum running around
Equipment: a Newton’s cradle ($18)
Newton’s cradle is a set of 5 steel balls hanging by light monofilament line. At rest, all 5 balls lie in a straight line, touching each other. One can then take any number of balls away to the side and release them, in order to watch the responses of all the balls, both those released and those initially at rest, They collide, move apart, collide again and again, while energy losses slow them (we’ll talk about where those losses are, later).
Newton’s cradle
Static
Videos for frame grabs – 1L; 2L; 3L; 1L+1R; 1L + 2R; 1+2L to stick to 3
The object lesson is learning the conservation of momentum, as well as the conservation of energy. Moving objects carry momentum; that’s the quantity of motion and it equals the mass of the object multiplied by its velocity. Yes, velocity has direction, not only magnitude or size, and so does momentum, but we know we’re only talking about motion in one direction, so let’s just call it speed. With mass m and speed v, the momentum is calculated simply as mv, that is, m multiplied by v.
The object also carries energy of motion, called kinetic energy. You need a bit more physics, but let’s just use the result that kinetic energy, KE, equals ½ the mass multiplied by the square of the speed, or ½ mv2.
When “elastic” objects such as steel ball bearings collide in the Newton’s cradle, momentum and energy both go into other balls. This tells us what the pattern of motion is after the collision.
Consider lifting the leftmost two balls up and to the left, then releasing them. They hit the three stationary balls. Then, the ball in the middle stays put and the two rightmost balls fly off to the right, a mirror image of the starting case. Those two balls are then moving at the same speed as the leftmost balls had when they contacted the other balls. It’s easy to prove mathematically that the only state of motion that conserves momentum and that also conserves kinetic energy is this fling to the right by two balls. You won’t get all three originally stationary balls moving off at various speeds; only two balls move. (The exact solution in physics gives a slightly different result, but minimally different when we take account of the tiny starting separations of the balls and the elastic deformations of the balls; yes, the steel balls deform a tiny bit as they collide! And, yes, steel is elastic, meaning that it deforms under a force and then regains its form almost exactly as the force is released. It’s a bit more complex that that, but steel and other elastic items don’t lose much energy in being hit and deformed.)
PICTURES – see list at top
Everyone likes to try different combinations – release only one ball from the left (only one ball leaves on the right); release three balls from the left (three balls leave on the right); release balls from opposite sides, one from the left and one from the right, both at the same speed (they both simply bounce back); release one ball from the left at twice the speed of a ball released from the right (check this out yourself). You can also make balls act as single units. Put a bit of modeling clay between two balls at the left and release them as a single unit. (Two balls shoot off to the right, the same as if the balls were not stuck together.) Try releasing those two balls at the same time as releasing a ball from the right at the same speed…. and then at twice the speed. Try putting clay on the side of the two balls that will hit the three stationary balls. What happens? You can see the results. If you want to get into the math, you can analyze all these cases. You could go to the exact solution, but that’s pretty heavy-duty math.
The exchange of momentum is very clear. The exchange of energy is a deeper story. In fact, we have to consider a second kind of energy called potential energy. In moving balls to the left or to the right while holding them “tight” on their supporting strings, we have to lift them up a bit against the force of gravity. That is storing potential energy. As the balls move, the potential energy is continuously converted into kinetic energy; gravity pulls on the balls to increase their movement, or accelerate them. The pattern in time of how potential energy changes into kinetic energy is an advanced topic that you’ll find in physics courses; the math is fun.
The motion of the balls slows down over time. Clearly, energy has been lost, both as kinetic energy and as potential energy. Where did it go? It got converted into two other forms. One is kinetic energy of air molecules pushed around as the balls move – they’re creating a little wind that carries energy farther and farther away. A second one is heat in the balls. Steel is not perfectly elastic. At each collision, some of the obvious or macroscopic motion gets changed into rapid internal motions among all of its atoms -sound waves run around in the ball and then slowly degrade into random motions called heat. You would find it vey hard to measure the rise in temperature of the balls after collisions. It’s very tiny, but real.
The challenge with the Newton’s cradle is that everyone wants to try their own releases. Leave more time for this than for most demos! It can be a good learning experience for students to sketch the resuls of all the different trials.