MANY IMAGES TO COME
Mostly physics, with a foray into biology
Fun with very cold things – dry ice or liquid nitrogen. Here are ten things to try, from making a flower shatter to liquefying air in a balloon to finding out how much water vapor you lose in your breath. Some you can do with inexpensive dry ice, some need liquid nitrogen (cheap, but the container is not!), and for all of them, take precautions. Here’s the link for the full set of stories.
Fun with very cold things – dry ice or liquid nitrogen
Equipment and supplies: cheap, unless you opt for a Dewar flask to hold liquid nitrogen ($300), but it’s useful lifelong; thermal insulating gloves are a must ($20)
Precautions: Frostbite damage to yourself is possible; be careful! Concentrated carbon dioxide from sublimating dry ice is toxic. Wear goggles for many of these demos. Don’t let liquid nitrogen stand a long time exposed to air to collect liquid oxygen.
The teaser: Many things act very differently when very cold – a flower shatters, as does a marshmallow, or even some steel. A balloon shrinks to nothing in liquid nitrogen (LN2) but reappears on warming. Make a witches’ caldron, or fog rings. Trap the water vapor in your breath. Make instant ice cream.
Picture Dewar; gloves; tongs
Images grabbed from 2018 demo at B&N?
Which items you can make depends in some cases on which ultracold source you choose. I describe these two chillers here and spell out precautions before getting to the fun:
* Dry ice is cheap (about $2/pound at a supermarket) and cold, at -78.5°C (-109°F). Many of the demos above can use dry ice, though not the disappearing balloon or simple instant ice cream. You can keep it in an insulated picnic cooler, and even transport it in a heavy paper bag. BE CAREFUL: besides its threat of freeze-burns, dry ice sublimates from solid to gas, without melting, generating abundant carbon dioxide gas, or CO2. CO2 concentrations above 10% are lethal with a number of breaths, so never keep dry ice in a small closed space where you might breathe in the CO2.
Dry ice will keep in a picnic cooler for a number of hours.
* Liquid nitrogen is cheap (about $2/liter at a welding supply store), but you must have a hyperinsulated Dewar flask, like a super Thermos bottle, to hold it; suppliers will only dispense it into a Dewar, as far as I am aware. It is hyper-cold, at -196°C, or only 77 kelvin above absolute zero; it’s at -320°F! It is the cldest thing you will experience in your entire life. In some ways, it’s safer than dry ice, in that you can’t get a solid piece stuck to your hand to continue freezing you. You may even see demos in which a person sticks his or her hand quickly into LN2 and then out again, with no ill effect; LN2 flashes into nitrogen gas that’s a fair thermal insulator. Don’t do it! Nitrogen gas is not toxic; it’s a simple asphyxiant, displacing oxygen, and you can readily recover with a new breath of ordinary air, provided that you haven’t passed out from a bad design of inhaling only high levels of N2 gas for awhile.
LN2 will keep for as much as 5 days in a good Dewar flask.
* Both dry ice and LN2 can “burn” your tissues from sufficient exposure – that is, for a sufficient time, though this is only several seconds, certainly for dry ice. USE HEAVY INSULATING GLOVES. Do not use gloves made of material that is porous. You can get good thermal gloves at a hardware store or a welding supply store.
* One final note, for an unlikely situation: nitrogen boils below the temperature at which oxygen condenses from air to form liquid oxygen or LOX. That’s a rocket propellant. If your demo device also has organic compounds in it where the LOX will accumulate, it makes an explosive situation. Avoid having organic compounds such as alcohol in the vessels you use for a demo. I was at Yale when a “trap” with only a fraction of a liter of LOX and organics exploded, breaking glass reagent bottles all over the lab.
The fun
Note: Wear goggles; some demos create flying small pieces
* Witches’ brew is classic, with dry ice. Fill a vessel with water. Break up a few chunks of dry ice and drop them in. A dense fog will form rapidly and hover above the water surface, while also spilling out onto the table or floor. It hovers and sinks because the fog is (1) cold and (2) containing a lot of CO2, which at any temperature is 50% denser than air. Low temperature alone makes a gas (vapor) denser, in inverse proportion to the absolute temperature. The vapor is only close to the freezing point of water, of course, not the temperature or dry ice, since it formed at the interface of the dry ice and water. You can make patterns in the fog, even mimicking those formed by airplanes traversing clouds or fog.
LN2 is much inferior in making witches’ brew. It’s less dense that water and floats on top, making an ice raft.
* Making soft items hard and fragile. A flower or a leaf is a structure made from water held under pressure in the plant cells. That pressurization is another interesting story (URL). Like a balloon, a water-filled cell is supple. You can mash it but not shatter it. That changes when you freeze it. A frozen flower or leaf shatters into fine piece when hit by, say, a hammer; then, all the pieces melt quickly to squishy things.
You can do the same demo with a marshmallow and then have tasty tidbits at the end.
You can do the same to a thin rubber ball, such as a handball; a thick or solid ball takes too long
Using LN2 to freeze an item: Have a handy container, such as a foam or paper cup (avoid metal cups that might tempt you to hold them; they’ll freeze to you, disastrously; avoid glass cups that might shatter from a rapid change in temperature). Pour some LN2 form the Dewar flask above halfway up the cup. WITH TONGS, hold the item and dip it into the LN2. It only takes a few seconds for a flower or a tin leaf. For a marshmallow or a handball keep it in the LN2 bath from15 to 30 seconds, waiting for the heavy boiling away of LN2 to slow (the boiling removes heat from the object). Remove the item and place it on a board or other solid surface (not glass, natch) and hit it sharply with a hammer. Voila!
Using dry ice to freeze an item: You need to make a medium that transfers heat well; you can’t readily hold a flower against a block of try ice and get it to freeze. The best and safest medium is pure alcohol, that is, ethanol. You might get some from a chemical supply store or, say, Carolina Biological Supply. Take care; there are legal constraints on having alcohol and there is a significant tax on the purchase. Ethanol freezes at -117°C (-180°F), so that it will stay liquid in contact with the dry ice and make a thick-ish slurry. Note that ethanol with some water will be a little messy, as the water will freeze out. Be careful handling large volumes of alcohol (pints, liters…); it is flammable; have a fire extinguisher handy and know how to use it. Break up the dry ice into dozens of small chunks and put them in a small insulated container. Pour on the alcohol and let it cool down until the frothing from CO2 subsides. Now use it as described for using LN2.
Acetone, or nail polish remover, also works as a heat transfer medium, as it stays liquid to -95°C (-139°F). However, do not use it unless you are a pro; it is very flammable, and more toxic than ethanol as a vapor.
An odd-on, that’s harder to do: making steel brittle: You can find hard steel and freeze it and then shatter it with a hammer. Surprisingly, case-hardened steels seem to be more vulnerable that cheaper steels. That’s why bike thieves can use liquid fluorocarbons as in Dust-Off to freeze and shatter a bike lock. Pick a thin piece of steel so you won’t end up hammering pointlessly. Hammer on a heavy wooden block so that you don’t damage the table or the floor.
* Quick ice cream: a nice quick freeze of milk or cream with sugar and flavoring makes fine crystals of ice, giving ice cream a fine, creamy texture. There’s no quicker way to freeze milk or cream than with LN2 (dry ice is not quite as good, we’ll see). In a wide container of an appropriate size, say, an empty icer cream container, make up a batch or milk, cream, or a mixture of the two; add sugar to taste (say, ¼ a much volume as the dairy product) and flavoring such as vanilla extract (chocolate is harder to deal with). WEARING your thermal gloves and goggles, pour in about a bit more than twice that volume of LN2 from the Dewar and stir quickly, vigorously, and continuously for about a minute, until the LN2 has evaporated. If you did it right, you’ll have nice and cold ice cream. Test the temperature before giving it to anyone to eat. If you used too much LN2 it may be so cold as to freeze-burn one’s tongue.
You’ll find it a bit tricky to measure LN2. It boils and churns as you pour it into anything at room temperature. Here’s a good way: get an insulated cup with a capacity of about 300 ml (more than 1 cup fluid measure). Let’s assume you’re making ½ cup of the milk mixture, or 120 ml. You’ll want about 264 ml of LN2, so, before you start, measure 264 ml of water into the cup and mark the level. Pour out the water and then use the emptied cup, filling it with LN2 to the mark. Pour that, slowly, into the milk mixture.
You can use a food mixer to keep the nascent ice cream churning as you add LN2. It also introduces some air bubbles that make an even nicer texture.
You can do the same process with dry ice. Break it up into fine chunks. You can figure out how much dry ice is needed per volume of cream and sugar mixture, using the heat of sublimation of dry ice… or you can just use 3/4 the volume of the cream and sugar mixture. That is, if you used 120 ml (1/2 cup) of milk/cream/sugar/flavor mixture, use 90 ml crushed dry ice. That is, fill the ½ cup measure ¾ full, or use a graduated cylinder, if you have one. The ice cream made this way will have a “bite,” an acidic taste from the CO2 that dissolved into the mixture to make some (harmless) carbonic acid. BE SURE that all the dry ice bits have sublimated away before serving the ice cream to anyone.BE SURE also that the ice cream is not too cold so as to cause frostbite to the lips or tongue.
* Blow up a balloon without breath: Both dry ice and LN2 undergo a change of phase from solid (dry ice) or liquid (LN2) to gas, with an enormous expansion in volume – over 1000-fold. You can put either one into a plastic bottle (not glass, certainly not for LN2) with a narrow mouth. Put a balloon over the mouth and watch it expand.
Make it much faster by adding water.
Make the volume change dramatic by using a tiny bottle to blow up a big balloon.
* Make smoke rings: Use a small squeezable bottle with a cap. Put a small hole in the cap with a needle or an awl (careful!). Fill the bottle with a bit of dry ice and water (or, less handily, with LN2 and water). Squeeze the bottle sharply to have it emit perfect smoke rings.
* Collapse a balloon to near-zero and let it expand again… and repeat it at will: Again using gloves and goggles and simple care, fill a foam or plastic cup with LN2 about halfway. Blow up a very long, narrow balloon, like the kind used to make balloon animals. It might start out 40 cm long (16”). Slowly immerse it into the LN2. It will shrink and shrink and shrink, to a flat piece! Be sure to use tongs to hold the tip at the end. Now remove the balloon and it will reinflate! Two effects are at work here. One is that gases contract in volume as the temperature is lowered. The great bulk of the effect is that the air in the balloon liquefies, to a mixture of LN2 and LOX, taking up less than a thousandth of the original volume.
* Really quick show: skittering droplets of LN2: Be sure no one has exposed skin near floor height; that means no open-toed shoes, for one. Take a modest quantity of LN2 in a cup and pour it onto the floor. Hundreds or thousands of tiny droplets will race across the floor, spreading out in an expanding set of tracks. Expect shrieks galore.
The spilled LN2 naturally breaks up into droplets, but these have no tendency to stick together. The LN2 in contact with the warm floor immediately flashes into gas. This has several effects. One is that it provides a bit of insulation so that the droplets stick around for many seconds. Another is that the little layer of gas beneath the droplet is a lubricant; the droplets float on it and maintain a notable speed. You can look up more under the term Leidnefrost effect. It’s the effect seen when you drop water onto a very hot pan that’s at a temperature way above the boiling point of water.
* This one is more of an experiment, beyond a simple demo: catch the water vapor in your breath: Get a length of food grade Tygon (plastic) tubing at a hardware store, about 2 m (6+ feet). Coil the central half of the tubing into a tight coil, about 10 cm (4”) in diameter and hold it coiled with long twist ties or string, your choice. Set up that bath with dry ice and alcohol, one that’s deep enough to submerge the coil. Have a person breathe in at a normal rate and volume but exhale through the tubing. Continue this for about 2 minutes. Remove the tubing from the bath and you’ll see water droplets in it. Uncoil the tubing and shake the droplets together. Estimate their volume, or cut the tubing and weigh the single drop with a common lad scale (about $16).
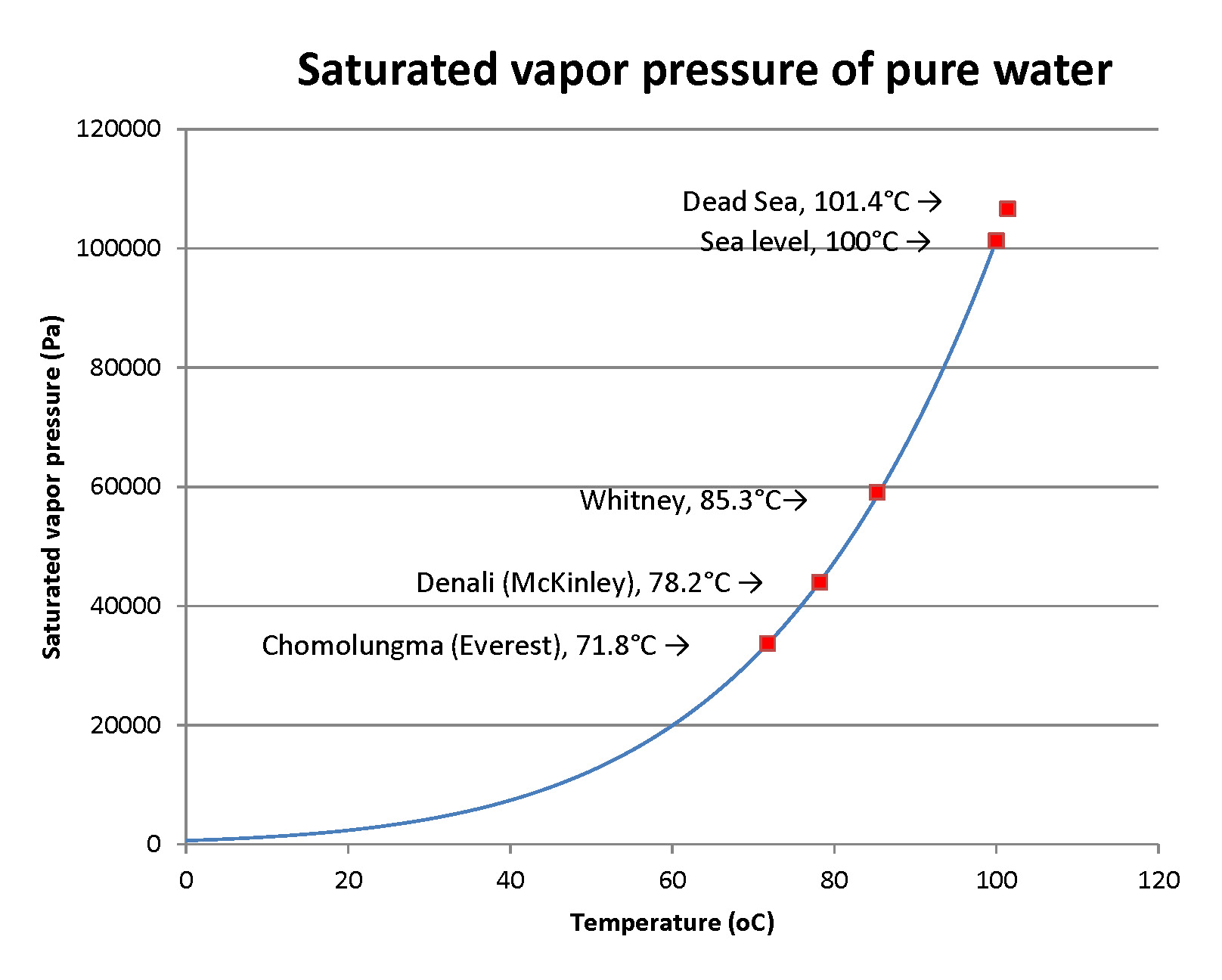
It may be surprising to the person that so much water was exhaled, as the gas water vapor. Here’s an estimate of how much to expect. In 2 minutes a person may take 12 breaths, each with a volume of about 0.5 liters. That’s 6 liters. Now, the amount of water vapor in the breath is the amount that is called saturating – the max that air can hold – at the temperature of the lungs. That temperature is close to core body temperature. That’s close to 37°C. There are nice mathematical formulas to compute this saturated water vapor pressure. In scientific units that’s 6300 pascals. It makes the air about 6% water vapor! Now we need some “gas laws” from physics. A short calculation yields the result that 6 liters of air at 37°C is a quantity of matter (air) that we can state as about ¼ of a mole of air (you’ll run into moles in chemistry). Six percent of ¼ of a mole is 0.015 mole. As a volume of water that’s ¼ of a cubic centimeter. It’s just measurable, so, does that make it not dramatic? The person who breathes a whole day breathes for 1440 minutes or about 720 times more than this sample. That makes the water loss in breathing about 720 * (0.25 cm3) or 180 ml. That’s easy to make up by drinking liquids. Well, at home, that is. If you’re climbing Chomolugma (Mt. Everest), really laboring and breathing many times the normal rate, you’ll lose much more… and you don’t want to carry that water. Better, use your stove to melt ice!